Abstract
Introduction.Ruxolitinib (RUX) is a JAK1/2 inhibitor able to control myelofibrosis (MF)-related splenomegaly and symptoms and improve overall-survival (OS). It is well known that long-term RUX-therapy at optimal doses contributes to clinical benefits, but impact of RUX-response feature on outcome is still relatively unknown.
Aims. To report the impact of response feature on clinical outcome of 356 patients (pts) with WHO-defined MF, INT-2 or high IPSS risk, treated with RUX.
Methods. A clinical database was created in 20 Italian Hematology Centres. Retrospective data on 356 MF pts treated with RUX according to prescribing information from Jan 2011 to Jun 2016 were collected. Response was defined as either one of the following at 3 (3M) and 6 (6M) months after RUX-start: reduction of splenomegaly 35% or reduction of MPN-SAF total symptom score-TSS 50% or ≥2.0 g/dL increase in hemoglobin (Hb) level. Pts were classified into four group of response: a) pts with stable response at 3M and 6M (sR); b) pts with stable non-response at 3M and 6M (sNR); c) pts with only early response at 3M (oER); pts with only late response at 6M (oLR). Survival was calculated from the start of RUX. Event-free survival (EFS) included progression to acute leukemia (AL), death and RUX discontinuation. In group comparison, the landmark approach was used at month 6 to eliminate guarantee-time bias.
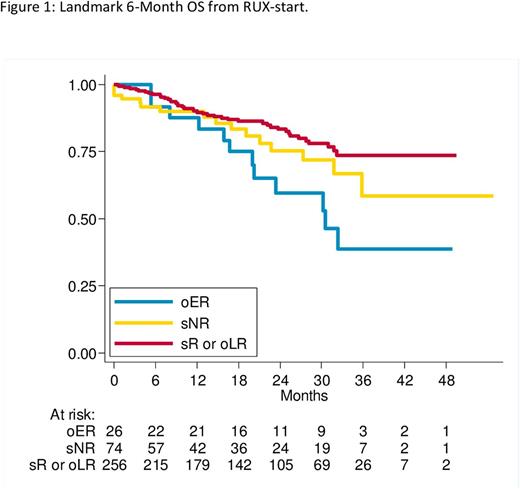
Results. A total of 356 (54% primary, 46% secondary to polycythemia or thrombocythemia) MF-patients were analyzed. Median age was 67 years (range 26-89) and 61% pts had a palpable splenomegaly at > 10 cm below left costal margin with a median spleen length of 11 cm. Median value of Hb and MPN-TSS were 11.1 g/dL and 20%, respectively. Median time from diagnosis to RUX-start was 16 months and median duration of RUX-therapy was 23 months. After a median of 27 months of follow-up from RUX-start, 244 patients (69%) were still receiving RUX-therapy, with 3y OS of 75% (70 deaths) and 3y EFS of 57% (16 deaths, 16 progressions to AL, 84 RUX discontinuation). Among the four group of response, we observed 229 sR, 74 sNR, 26 oER and 27 oLR. Using a 6-month landmark approach, 3y OS according to different group of response was 74%, 58%, 39%, and 70% for sR, sNR, oER and oLR, respectively. Using a Cox model adjusted for age and gender, patients oER showed a significantly worse OS respect to sR or oLR patients (HR=2.11, p=0.025) (Figure 1).
Discussion. In MF-patients a RUX-response at 6M (both stable response and only late response) seems to be associated with a clinical benefit in terms of OS. Despite the cohort of pts will be certainly further enriched, these preliminary data suggest the need to continue RUX-therapy for at least 6 months.
Bonifacio: Incyte: Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Tiribelli: Incyte: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Abruzzese: Novartis: Consultancy; Incyte: Consultancy; Pfizer: Consultancy; BMS: Consultancy. Cavazzini: Incyte: Consultancy; Bristol-Meyers Squibb: Consultancy; Novartis: Consultancy; Pfizer: Consultancy. Crugnola: BMS: Honoraria; Celgene: Honoraria; Novartis: Honoraria. Vitolo: Mundipharma: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cuneo: Gilead: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Abbvie: Honoraria, Other: Advisory Board; Roche: Honoraria, Other: Advisory Board. Breccia: Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy; Incyte: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal